Areas of Focus
The following are indications using PanCytoVir™:
COVID-19 (SARS-CoV-2)
The ongoing pandemic caused by SARS-CoV-2 has caused a global health challenge and has affected over 200 countries with over 11 million cases since 2020.
Respiratory viruses are the most frequent cause of disease in humans, having significant impacts on morbidity and mortality worldwide [21–23]. The most common respiratory viruses are endemic agents such as coronaviruses (CoVs), respiratory syncytial viruses (RSVs), and influenza (flu) viruses. Although vaccines are available for CoV2 and flu, there is a paucity of effective antiviral drugs. For RSV, there is no vaccine available, and therapeutic treatments are very limited.
The ongoing pandemic of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has plagued so far over 200 countries and has resulted in over 11 million cases and 500,000 deaths since the start of 2020.
COVID-19 has accentuated the need for drug discovery, and this has helped propel the need for a better understanding of virus-host cell interactions, with the hope that this
could facilitate drug breakthroughs.
Effective vaccines are slowing the COVID-19 pandemic, but SARS-CoV-2 will likely remain an issue in the future making it important to have therapeutics to treat patients. There are few options for treating patients with COVID-19. We show PanCytoVir™ potently blocks SARS-CoV-2 replication in mammalian cells and virus replication in a hamster model. PanCytoVir™ is an FDA-approved drug with a well-documented (>7 decades) safety profile for treating gout.
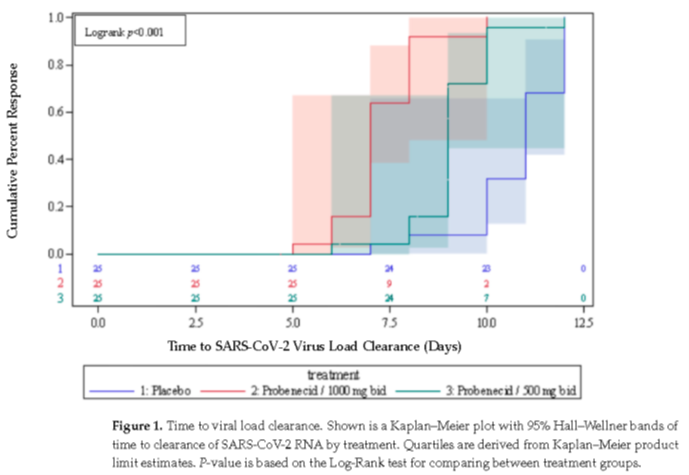
More recently, we reported the results of a phase 2 randomized, placebo-controlled, single-blind, dose-range finding study in non-hospitalized patients with symptomatic, mild-to-moderate COVID-19. Patients were randomly assigned in a 1:1:1 ratio to receive either 500 mg of probenecid, 1000 mg of probenecid, or a matching placebo every 12 h for five days. The patients’ COVID-19 viral load hospitalization, or death from any cause through day 28, as well as safety, were evaluated. COVID-19-related symptoms were assessed at baseline, and on days 3, 5, 10, 15, and 28. The primary endpoints of the study were time to first negative SARS-CoV-2 viral test (or viral clearance) and the proportion of patients that were symptom-free at day 5. A total of 75 patients were randomized, with 25 patients in each group. All of the patients completed the study as planned with no hospitalizations or deaths being reported. The median time to viral clearance was significantly shorter for the probenecid 1000 mg group than for placebo (7 days vs. 11 days, respectively; p < 0.0001), and for the probenecid 500 mg group versus placebo (9 days vs. 11 days, respectively; p < 0.0001). In addition, the median time to viral clearance was significantly shorter for the probenecid 1000 mg group than for the probenecid 500 mg group (7 days vs. 9 days, respectively; p < 0.0001). All patients reported at least one COVID-19-related symptom on days 3 and 5; however, on day 10, a significantly greater proportion of patients receiving probenecid 1000 mg reported the complete resolution of symptoms versus placebo (68% vs. 20%, respectively; p = 0.0006), as well as for those receiving probenecid 500 mg versus placebo (56% vs. 20%, respectively, p = 0.0087). The incidence of adverse events during treatment was similar across all groups for any adverse event, and was 12%. All events were mild with no serious adverse events reported and no discontinuations due to an adverse event. The treatment of patients with symptomatic, mild-to-moderate COVID-19 with probenecid resulted in a significant, dose-dependent decrease in the time to viral clearance and a significantly higher proportion of patients reporting complete symptom resolution by day 10.
Discussions are currently underway with the FDA on the design and timing for the Phase 3 program.
Quoted Research Articles
Martin DE, Pandey N, Chavda P, Singh G, Sutariya R, Sancilio F, and Tripp RA. Oral Probenecid for Nonhospitalized Adults with Symptomatic, Mild-to-Moderate COVID-19. Viruses 2023:15;1508. https://doi.org/10.3390/v15071508
Influenza
Influenza type A viruses are categorized based on the combinations of two different proteins: the hemagglutinin (H) and the neuraminidase (N), located on the surface of the virus. The currently circulating type A viruses are H1N1 and H3N2 subtypes. Type A viruses cause pandemics. Currently circulating type B viruses are of two main groups: B/Yamagata and B/Victoria lineages.
Influenza A and B are outbreak-driven, and thus there is no prevalence pattern. In temperate climates, seasonal outbreaks occur mainly during winters, while in tropical regions, influenza may occur throughout the year. Influenza is termed an “unpredictable threat.”
In 1997, WHO launched a global web-based tool for influenza virological surveillance where data from National Influenza Centers (NICs) of the Global Influenza Surveillance and Response System (GISRS) and other national influenza reference laboratories collaborating with GISRS are used to track the spread of influenza virus. The tool monitors the evolution of influenza viruses and provides recommendations in
laboratory diagnostics, vaccines, antiviral susceptibility and risk assessment. It also serves as a global alert mechanism for the emergence of influenza viruses with pandemic potential.
The ongoing COVID-19 pandemic has an impact on influenza.
The pandemic has influenced to varying extents health-seeking behaviors, staffing/routines in sentinel sites, as well as testing priorities and capacities in Member States. The various hygiene and social distancing measures implemented by Member States to reduce SARS-CoV-2 virus transmission have also contributed in reducing influenza virus transmission.
PanCytoVir™ Activity
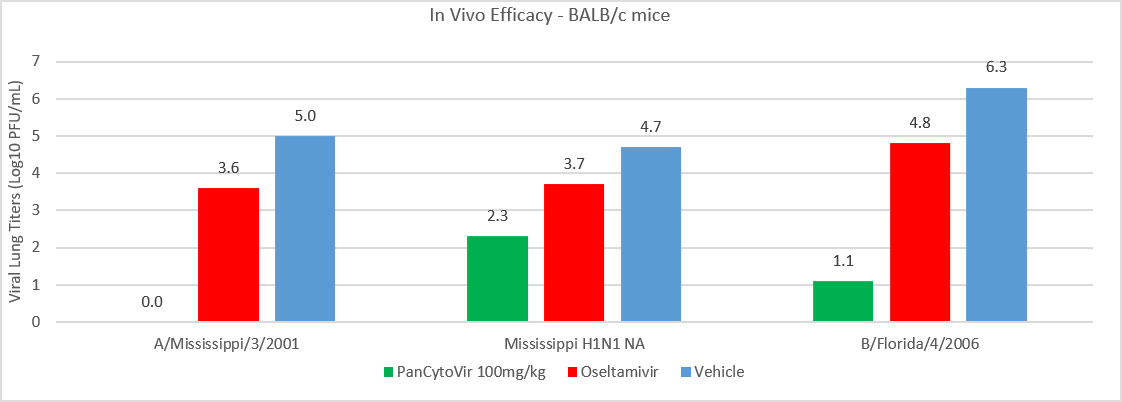
PanCytoVir has been shown to be a broad-spectrum antiviral agent with a novel mechanism of action. The antiviral activity of PanCytoVir™ against influenza is more potent, in vitro, than Tamiflu® against contemporary influenza A and B strains, H7N9 avian influenza A and H5N1, a highly pathogenic influenza A virus. The potency difference was also observed in vivo with both A and B strains. Recent data in patients with symptomatic, mild-to-moderate COVID-19 showed that PanCytoVir™ treatment significantly reduced SARS-CoV-2 viral load, and significantly more treated patients had complete resolution of COVID-19-related symptoms by Day 10 versus placebo. This is important as the antiviral mechanism of action against SARS-CoV-2 is shared with influenza, suggesting an increased probability of success in clinical studies.
Figure 1. Female 6-8 week old BALB/c mice (n=5/group) were infected with A/Mississippi/3/2001, A/Mississippi/3/2001 H275Y, or B/Florida/4/2006. Lungs were processed and titered on MDCK cells at day 5 pi. PanCytoVir reduced viral load the greatest throughout all three studies. Note: PanCytoVir OG 100mg/kg reduced viral load completely so these values are zero and not visible on the graph.

RSV
It is reported in a recent article published in Open Forum Infectious Diseases journal, 2021, that the global burden of RSV study of 2016, estimated that RSV is responsible for 24.8 million acute respiratory tract infections (ARI) episodes and 76,600 deaths each year. Nearly 60%-70% of the children below the age of one have been infected with RSV and 2%–3% of these infections result in hospitalization.
RSV is a leading cause of mortality and morbidity in children aged below five years, particularly in low- and median-income countries. The incidence of RSV is lower in adults, however, it has been increasingly recognized as an important cause of respiratory disease in adults. The articles also have data from 15 participating countries which report the median cases per season and the care level (hospitalized or community care). The data from the 15 countries found that the majority (55%) of RSV cases occurred in the <1-year-olds, with 8% of cases reported in those aged ≥65 years. Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract disease in children <2 years of age. Increased morbidity and mortality have been reported in high-risk patients, such as premature infants, patients with cardiac disease, and severely immune-compromised patients.
Transmission of the virus occurs through infectious respiratory secretions, such as coughing or sneezing. The virus can also live for hours on objects, so the infection can spread via this route as well. The virus enters the body of a new host through the eyes, nose, or mouth.
There are some groups of people who may be more at risk of contracting RSV, including young children and older adults. Interestingly, RSV does not induce long-term immunity and people can be repeatedly infected. RSV infections are continually a cause of morbidity and mortality around the world in infants, elderly, and high-risk patients. Mainstream therapy remains restricted to supportive care.
RNA viruses like SARS-CoV-2, influenza virus, and respiratory syncytial virus (RSV) are dependent on host genes for replication. PanCytoVir™, was evaluated for prophylactic or therapeutic efficacy to inhibit RSV replication in three epithelial cell lines used in RSV studies, i.e., Vero E6 cells, HEp-2 cells, and in primary normal human bronchoepithelial (NHBE) cells, and in BALB/c mice. These studies showed that nanomolar concentrations of all PanCytoVir™ regimens prevent RSV strain A and B replication in vitro and RSV strain A in vivo, representing a potential prophylactic and chemotherapeutic for RSV.
TD-214, a novel, new chemical entity that safely and efficiently delivers PanCytoVir™ into the bloodstream is currently being evaluated for use as a therapeutic treatment for RSV infection.